By: Ahlqvist, Grace P.; McGeough, Catherine P.; Senanayake, Chris; Armstrong, Joseph D.; Yadaw, Ajay; Roy, Sarabindu; Ahmad, Saeed; Snead, David R.; Jamison, Timothy F.
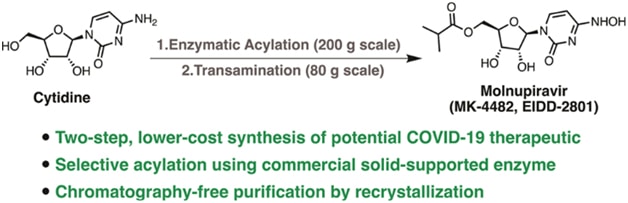
Molnupiravir (MK-4482, EIDD-2801, I) is a promising orally bioavailable drug candidate for the treatment of COVID-19. Herein, we describe a supply-centered and chromatog.-free synthesis of molnupiravir from cytidine, consisting of two steps: a selective enzymic acylation followed by transamination to yield the final drug product. Both steps have been successfully performed on a decagram scale: the first step at 200 g and the second step at 80 g. Overall, molnupiravir has been obtained in a 41% overall isolated yield compared to a max. 17% isolated yield in the patented route. This route provides many advantages to the initial route described in the patent literature and would decrease the cost of this pharmaceutical should it prove safe and efficacious in ongoing clin. trials.
ACS Omega
Volume6
Issue15
Pages10396-10402
Journal; Online Computer File
2021
CODEN:ACSODF
ISSN:2470-1343
DOI:10.1021/acsomega.1c00772